Oncogenic burden of obesity
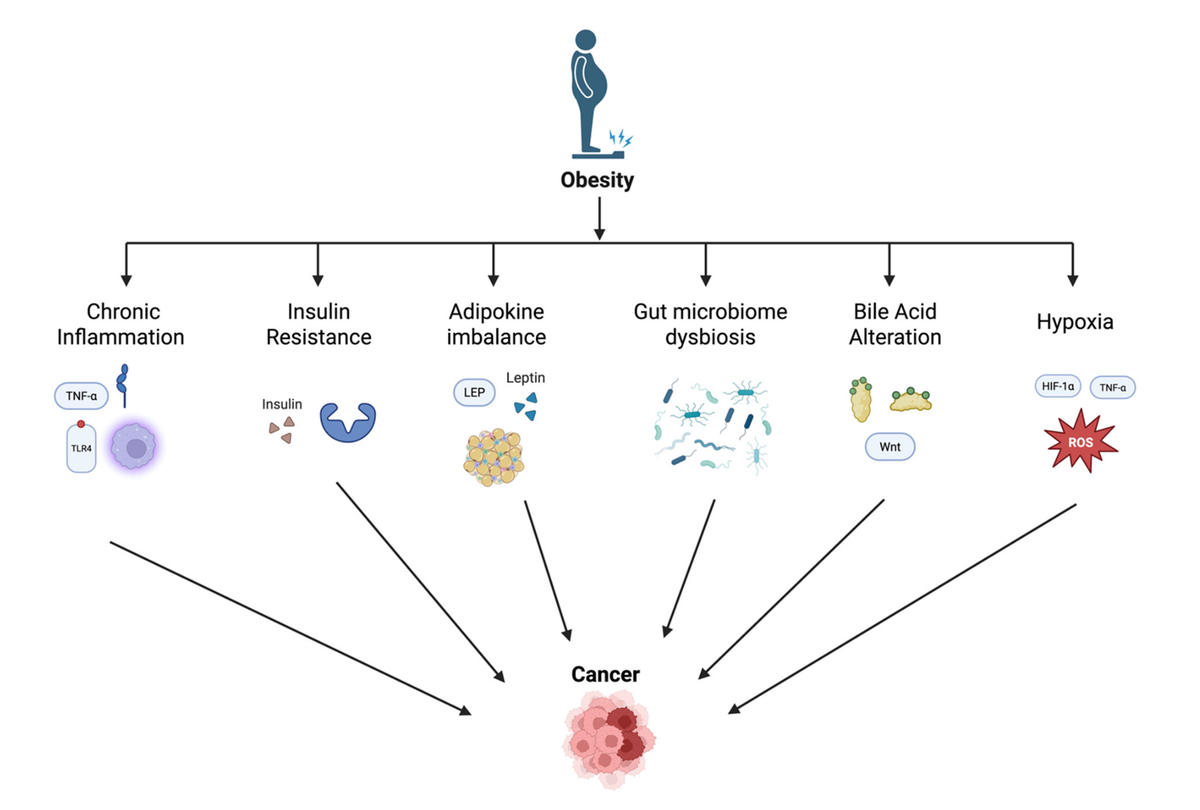
This article provides a mechanistic framework relating obesity to increased risk of a number of gastrointestinal cancers, including esophageal adenocarcinoma. It also highlights the potential of weight loss interventions (e.g., lifestyle, pharmacologic, surgical) to reduce GI cancer risk. The authors describe a complex interplay of metabolic, inflammatory, microbial, and genetic factors that drive carcinogenesis in these tissues (see Figure 1). Central to this connection, especially for esophageal adenocarcinoma, is chronic inflammation, induced in multiple ways by excess adipose tissue, which fosters an environment conducive to tumor initiation and progression. As a narrative review, this paper offers a broad and potentially subjective overview which is not necessarily comprehensive; rather it serves to steer translational research towards effective prevention and screening measures.
The Oncogenic Burden of Obesity: Mechanistic Links Between Adiposity and Gastrointestinal Cancers-A Comprehensive Narrative Review.
Lee F, Moore J, Markouli M, Ghusn W.
Abstract
Obesity is a global health crisis with profound implications for cancer risk, particularly within the gastrointestinal (GI) tract. Mounting evidence demonstrates that excess adiposity contributes to the initiation, progression, and poor outcomes of GI malignancies through a constellation of interrelated mechanisms. This review comprehensively examines the biologic pathways linking obesity to cancers of the esophagus, stomach, colon, liver, pancreas, and gallbladder.
Chronic low-grade inflammation, driven by adipose tissue-derived cytokines and immune cell infiltration, plays a central role in tumorigenesis via the activation of NF-κB, STAT3, and other pro-oncogenic signaling cascades. Hyperinsulinemia and insulin resistance increase mitogenic IGF-1 signaling, while dysregulated adipokines, particularly elevated leptin and reduced adiponectin, promote cellular proliferation and impair tumor suppression. Dysbiosis of the gut microbiome and alterations in bile acid metabolism generate carcinogenic metabolites that contribute to DNA damage and immune evasion. Additionally, obesity-induced tissue hypoxia fosters tumor growth through HIF-1α-mediated pathways. We further highlight organ-specific associations, such as visceral adiposity's role in Barrett's esophagus and hepatocellular carcinoma emerging from metabolic dysfunction-associated steatotic liver disease (MASLD).
Importantly, emerging data suggest that weight loss, achieved via lifestyle, pharmacologic, or surgical interventions, may mitigate these carcinogenic pathways and improve tumor biology. As obesity prevalence continues to rise globally, elucidating its mechanistic ties to GI malignancies is essential for risk stratification, prevention strategies, and personalized care. By integrating epidemiologic and molecular insights, this review underscores the need for multidisciplinary approaches to curb the oncogenic burden of obesity and improve outcomes in GI oncology.