Mathematical modeling suggests older ages for beginning screening
Kit Curtius and colleagues demonstrate how mathematical modeling of cancer evolution can be used to optimize age at initial screening for Barrett's and esophageal adenocarcinoma, suggesting an older start.
Cancer Res. 2021 Feb 15;81(4):1123-1134. doi: 10.1158/0008-5472.CAN-20-0335. Epub 2020 Dec 8.
Optimal Timing for Cancer Screening and Adaptive Surveillance Using Mathematical Modeling
Kit Curtius, Anup Dewanji , William D Hazelton , Joel H Rubenstein, Georg E Luebeck
PMID: 33293425 PMC7611607
Abstract
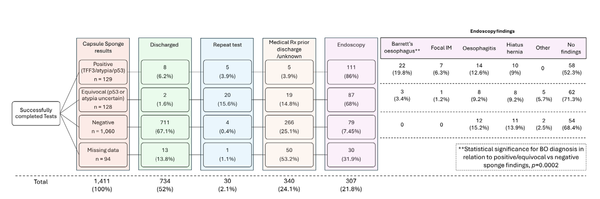
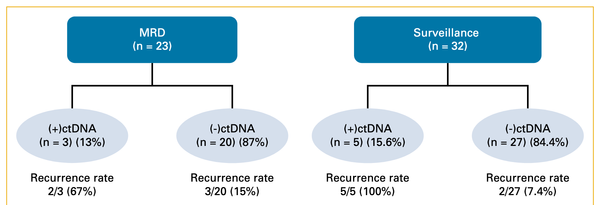
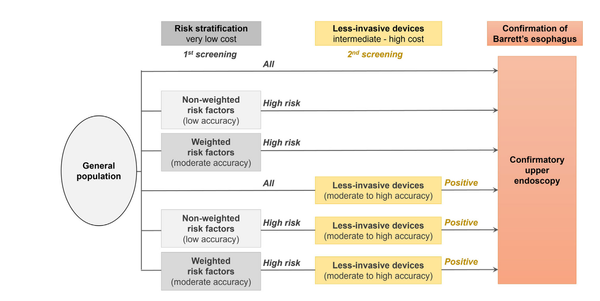
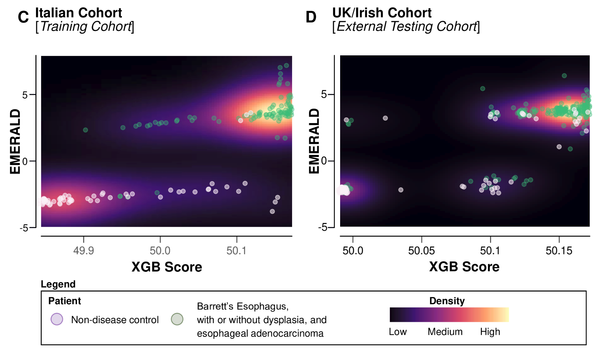
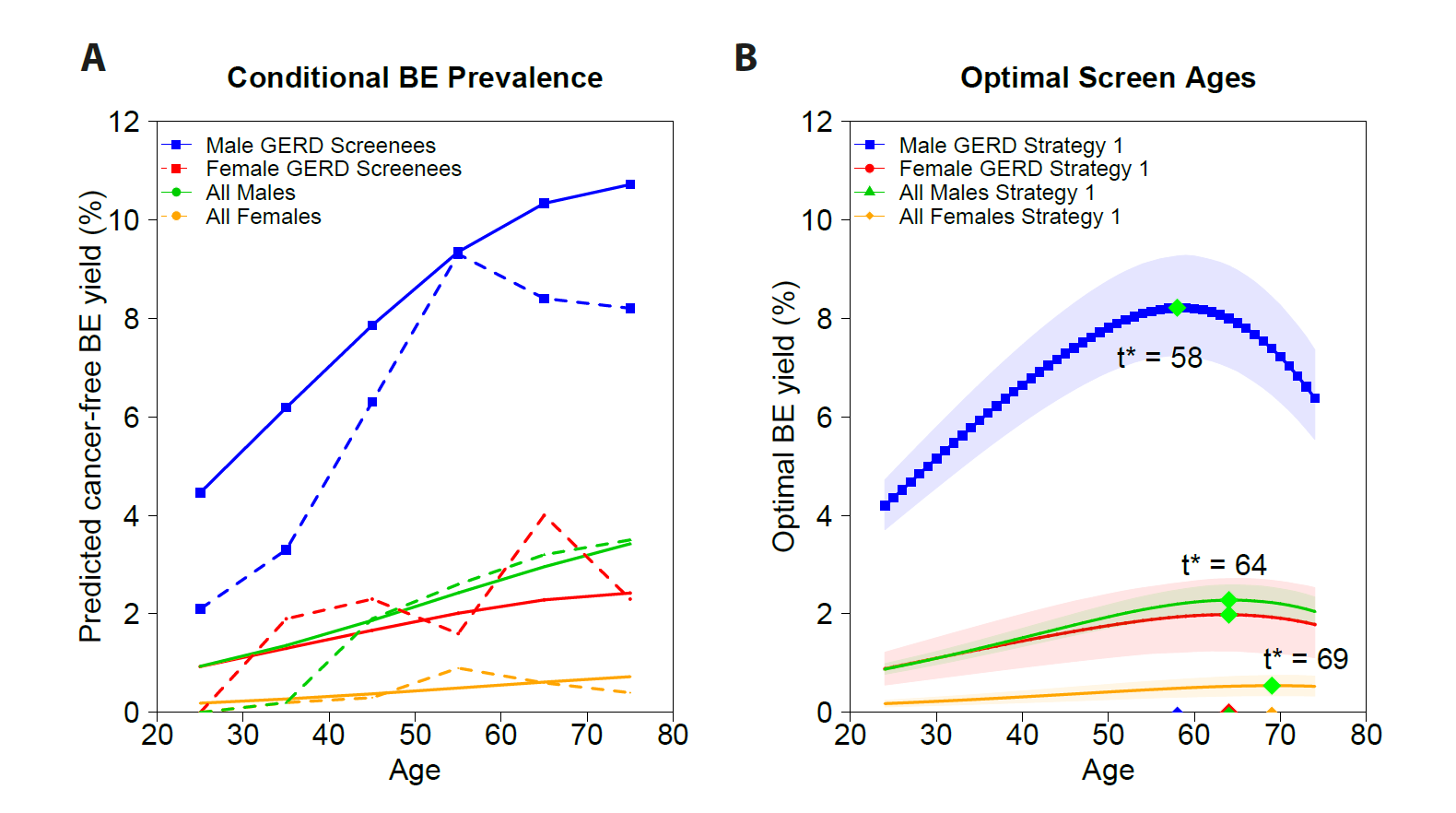
Cancer screening and early detection efforts have been partially successful in reducing incidence and mortality, but many improvements are needed. Although current medical practice is informed by epidemiologic studies and experts, the decisions for guidelines are ultimately ad hoc. We propose here that quantitative optimization of protocols can potentially increase screening success and reduce overdiagnosis. Mathematical modeling of the stochastic process of cancer evolution can be used to derive and optimize the timing of clinical screens so that the probability is maximal that a patient is screened within a certain "window of opportunity" for intervention when early cancer development may be observable. Alternative to a strictly empirical approach or microsimulations of a multitude of possible scenarios, biologically based mechanistic modeling can be used for predicting when best to screen and begin adaptive surveillance. We introduce a methodology for optimizing screening, assessing potential risks, and quantifying associated costs to healthcare using multiscale models. As a case study in Barrett's esophagus, these methods were applied for a model of esophageal adenocarcinoma that was previously calibrated to U.S. cancer registry data. Optimal screening ages for patients with symptomatic gastroesophageal reflux disease were older (58 for men and 64 for women) than what is currently recommended (age > 50 years). These ages are in a cost-effective range to start screening and were independently validated by data used in current guidelines. Collectively, our framework captures critical aspects of cancer evolution within patients with Barrett's esophagus for a more personalized screening design. SIGNIFICANCE: This study demonstrates how mathematical modeling of cancer evolution can be used to optimize screening regimes, with the added potential to improve surveillance regimes.
©2020 American Association for Cancer Research.