Prioritising BE surveillance using Cytsponge biomarker panel and clinical and epidemiologic risk factors
In addition to serving as a minimally invasive screening tool to help identify persons with Barrett's (BE), dysplasia and early stage esophageal adenocarcinoma, the Cytosponge (and similar devices) also have a potentially important role in long term surveillance of persons with BE.
Lancet Oncol. 2022 Feb;23(2):270-278. doi: 10.1016/S1470-2045(21)00667-7. Epub 2022 Jan 11.
Use of a Cytosponge biomarker panel to prioritise endoscopic Barrett's oesophagus surveillance: a cross-sectional study followed by a real-world prospective pilot
Nastazja Dagny Pilonis 1, Sarah Killcoyne 1, W Keith Tan 1, Maria O'Donovan 2, Shalini Malhotra 2, Monika Tripathi 2, Ahmad Miremadi 2, Irene Debiram-Beecham 1, Tara Evans 1, Rosemary Phillips 3, Danielle L Morris 4, Craig Vickery 5, Jon Harrison 6, Massimiliano di Pietro 1, Jacobo Ortiz-Fernandez-Sordo 7, Rehan Haidry 7, Abigail Kerridge 1, Peter D Sasieni 8, Rebecca C Fitzgerald 9
PMID: 35030332 PMCID: PMC8803607 DOI: 10.1016/S1470-2045(21)00667-7
Abstract
Background: Endoscopic surveillance is recommended for patients with Barrett's oesophagus because, although the progression risk is low, endoscopic intervention is highly effective for high-grade dysplasia and cancer. However, repeated endoscopy has associated harms and access has been limited during the COVID-19 pandemic. We aimed to evaluate the role of a non-endoscopic device (Cytosponge) coupled with laboratory biomarkers and clinical factors to prioritise endoscopy for Barrett's oesophagus.
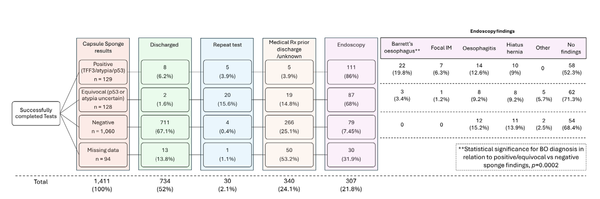
Methods: We first conducted a retrospective, multicentre, cross-sectional study in patients older than 18 years who were having endoscopic surveillance for Barrett's oesophagus (with intestinal metaplasia confirmed by TFF3 and a minimum Barrett's segment length of 1 cm [circumferential or tongues by the Prague C and M criteria]). All patients had received the Cytosponge and confirmatory endoscopy during the BEST2 (ISRCTN12730505) and BEST3 (ISRCTN68382401) clinical trials, from July 7, 2011, to April 1, 2019 (UK Clinical Research Network Study Portfolio 9461). Participants were divided into training (n=557) and validation (n=334) cohorts to identify optimal risk groups. The biomarkers evaluated were overexpression of p53, cellular atypia, and 17 clinical demographic variables. Endoscopic biopsy diagnosis of high-grade dysplasia or cancer was the primary endpoint. Clinical feasibility of a decision tree for Cytosponge triage was evaluated in a real-world prospective cohort from Aug 27, 2020 (DELTA; ISRCTN91655550; n=223), in response to COVID-19 and the need to provide an alternative to endoscopic surveillance.
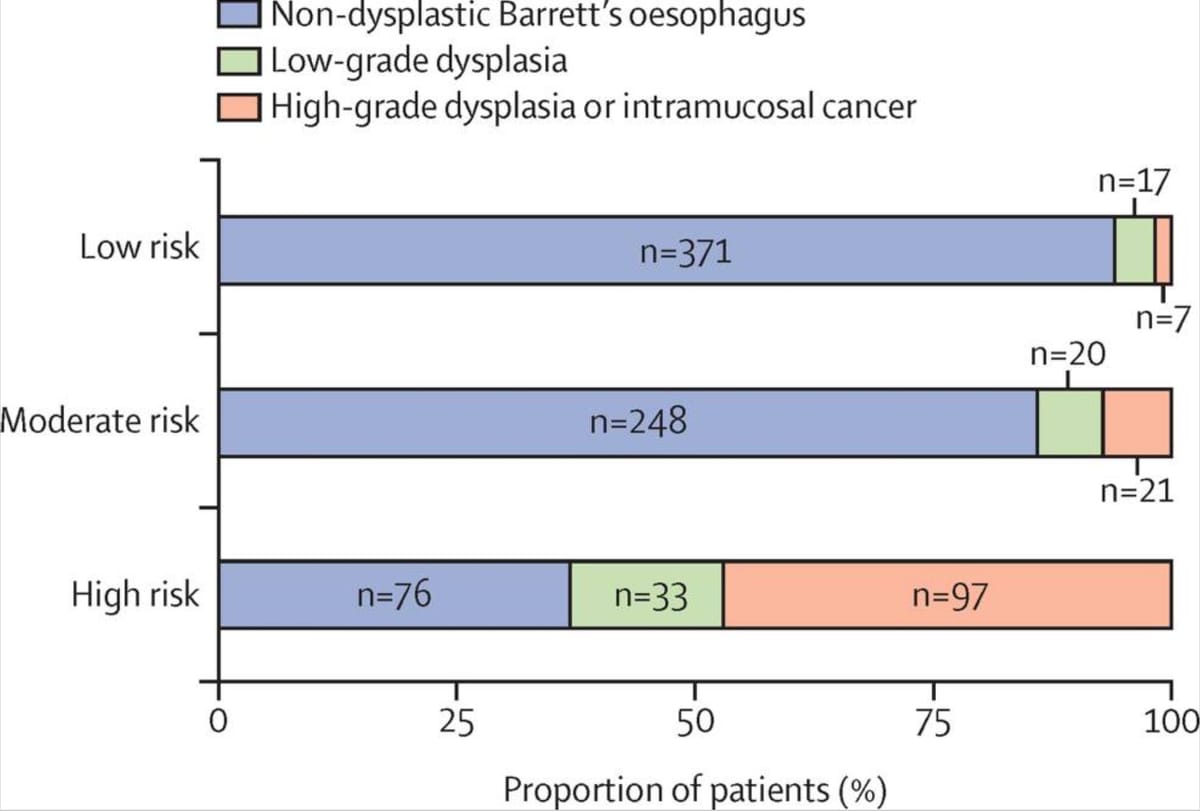
Findings: The prevalence of high-grade dysplasia or cancer determined by the current gold standard of endoscopic biopsy was 17% (92 of 557 patients) in the training cohort and 10% (35 of 344) in the validation cohort. From the new biomarker analysis, three risk groups were identified: high risk, defined as atypia or p53 overexpression or both on Cytosponge; moderate risk, defined by the presence of a clinical risk factor (age, sex, and segment length); and low risk, defined as Cytosponge-negative and no clinical risk factors. The risk of high-grade dysplasia or intramucosal cancer in the high-risk group was 52% (68 of 132 patients) in the training cohort and 41% (31 of 75) in the validation cohort, compared with 2% (five of 210) and 1% (two of 185) in the low-risk group, respectively. In the real-world setting, Cytosponge results prospectively identified 39 (17%) of 223 patients as high risk (atypia or p53 overexpression, or both) requiring endoscopy, among whom the positive predictive value was 31% (12 of 39 patients) for high-grade dysplasia or intramucosal cancer and 44% (17 of 39) for any grade of dysplasia.
Interpretation: Cytosponge atypia, p53 overexpression, and clinical risk factors (age, sex, and segment length) could be used to prioritise patients for endoscopy. Further investigation could validate their use in clinical practice and lead to a substantial reduction in endoscopy procedures compared with current surveillance pathways.
Funding: Medical Research Council, Cancer Research UK, Innovate UK.
Copyright © 2022 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.