Using the Cytosponge for targeted screening of Barrett's
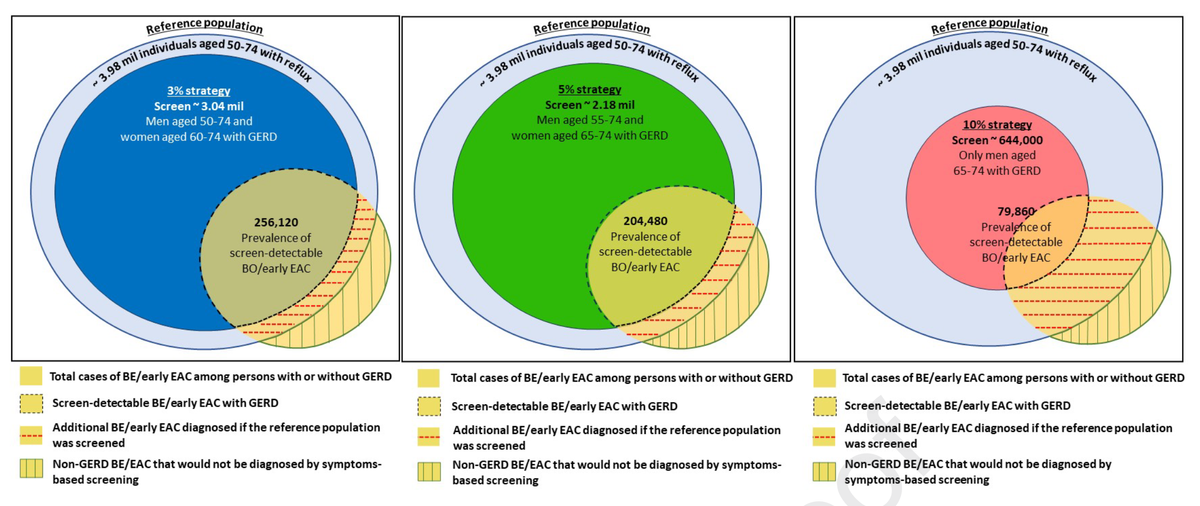
Using data from the previously published BEST3 clinical trial (1), the authors estimated the potential for improving the detection of Barrett's esophagus (BE) and early esophageal adenocarcinoma (EAC) among individuals aged 50 and above with gastroesophageal reflux disease (GERD). They calculated that the current referral strategies identify only a fraction (13/680, 2%) of the projected BE and stage-1 EAC expected among a reflux population aged ≥50. By targeting screening to individuals with a 5% probability of BE/EAC, the study suggests a substantial decrease in the population to screen while still detecting over 70% of cases among those with GERD. However, the authors acknowledge that the approximately 40% of BE/EAC with GERD would still be missed.
For reference, the risk calculator (IC-RISC) available on the ESOCAN website estimates 10-year risk of EAC (not BE) based on all of the major known risk factors (including GERD) from six studies in the BEACON consortium (2). Of note, positive results from non-endoscopic screening for BE, as in the BEST3 trial, can also be included in the present risk calculator, with likely substantial improvement in its positive predictive value.
1. Fitzgerald RC, di Pietro M, O'Donovan M, et al. Cytosponge-trefoil factor 3 versus usual care to identify Barrett's oesophagus in a primary care setting: a multicentre, pragmatic, randomised controlled trial. The Lancet 2020;396:333-344.
2. Vaughan TL, Onstad L, Dai JY. Interactive decision support for esophageal adenocarcinoma screening and surveillance. BMC Gastroenterol. 2019 Jun 27;19(1):109. doi: 10.1186/s12876-019-1022-0. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4382373/
Targeted screening for Barrett’s esophagus and esophageal cancer: Post-hoc analysis from the randomized BEST3 trial
Gastroenterology (2024), doi: https://doi.org/10.1053/ j.gastro.2024.04.030.
Tan WK, Maroni R, Offman J, Zamani SA, BEST3 Trial Team, Sasieni PD, Fitzgerald RC.
(no abstract)