Capsule sponge, biomarkers and AI in risk classification of Barrett's esophagus
This paper describes findings from a multicenter prospective study conducted across 13 hospitals in the UK. Participants were patients (n = 910) with a known diagnosis of non-dysplastic Barrett's esophagus who underwent a capsule-sponge (Cytosponge) test followed by endoscopy. The Cytosponge test combined with biomarker analysis, including p53 staining and glandular atypia, were used to stratify patients into low, moderate, and high-risk groups for developing dysplasia or cancer. A machine learning and AI model was trained and tested to evaluate its ability to reduce pathologist time in preparing for scaling up the surveillance method. The deep learning models were trained using slides from the BEST2 clinical trial. The study was powered to establish the safety of capsule-sponge surveillance in the low-risk group from a public health perspective.
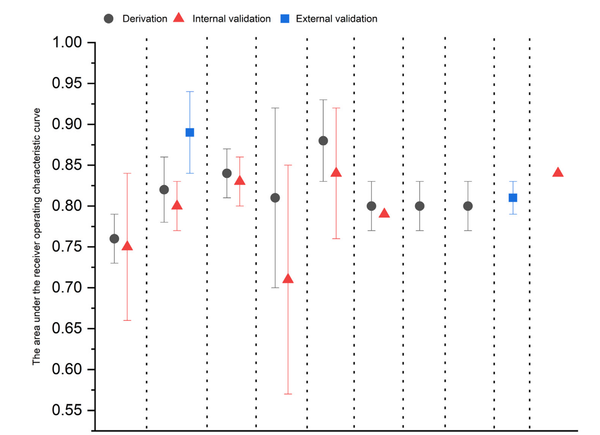
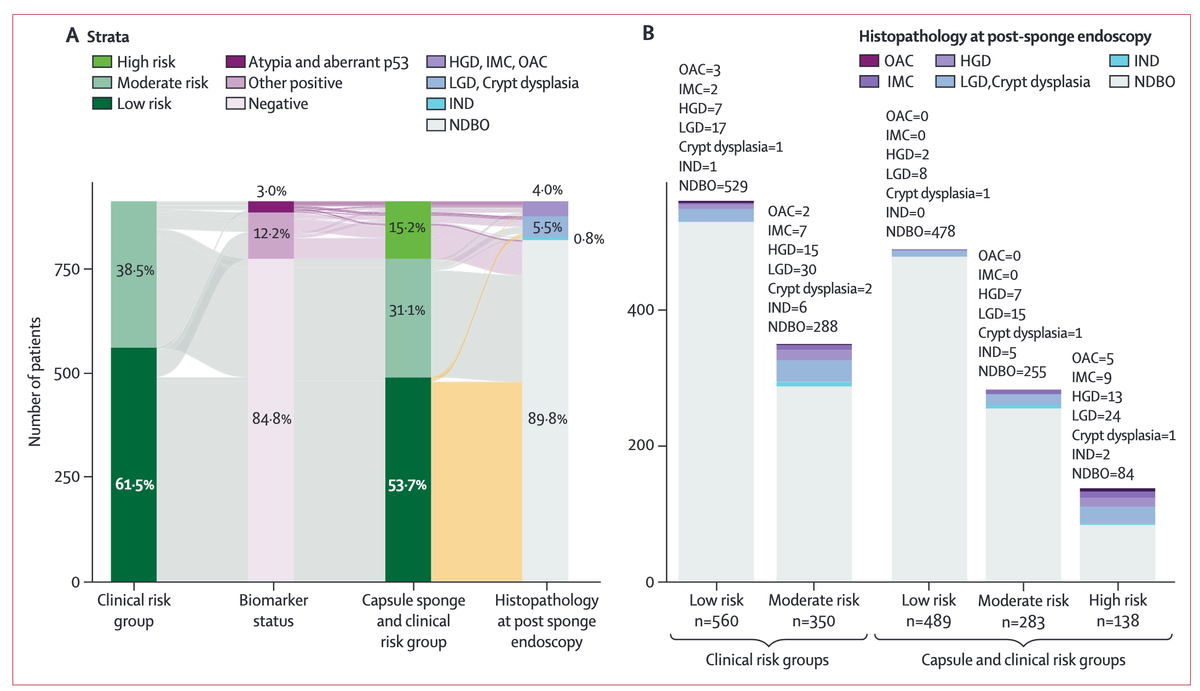
Notably, the authors report that in those patients classified as low risk, who constituted over 50% of patients, the subsequent prevalence of high-grade dysplasia or adenocarcinoma was 0·4% with a 95% CI below the clinical threshold for a primary care referral for endoscopy of 3%. Compared to this low-risk group, those in the high-risk category (15% of participants) experienced a 135-fold increase in prevalence of dysplasia or cancer. Some of the limitations include the lack of centralized pathology review and an unexplained higher-than-expected rate of dysplasia detection.
Overall, this paper presents several potentially significant contributions:
- It introduces and validates a multi-tiered (low, moderate, high) risk stratification system for BE patients combining:
- A non-invasive capsule sponge test for sample collection,
- Specific biomarkers (p53 overexpression and glandular atypia) analyzed from the sponge samples, and
- Clinical factors (segment length, sex, age).
- It integrates an AI/deep learning algorithm to analyze digital pathology images obtained from the sponge. This AI model:
- Assists pathologists in classifying glandular structures and detecting biomarkers like p53 overexpression,
- Demonstrates high sensitivity (e.g., 100% for p53 overexpression), and
- Aims to improve the efficiency, objectivity, and scalability of pathology review, potentially reducing the need for extensive manual pathologist review and enabling broader implementation.
- It demonstrates the practical applicability of this combined approach in a real-world clinical setting to effectively:
- Identify low-risk patients who can undergo sponge surveillance, potentially replacing some endoscopies,
- Prioritize high-risk patients for immediate endoscopy, significantly reducing wait times for those most in need, and
- Provide a scalable, more efficient diagnostic tool for widespread clinical practice.
Biomarker risk stratification with capsule sponge in the surveillance of Barrett's oesophagus: prospective evaluation of UK real-world implementation.
Tan WK, Ross-Innes CS, Somerset T, Markert G, Markowetz F, O'Donovan M, di Pietro M, Sasieni P, Fitzgerald RC; DELTA consortium.
Abstract
Background: Endoscopic surveillance is the clinical standard for Barrett's oesophagus, but its effectiveness is inconsistent. We have developed a test comprising a pan-oesophageal cell collection device coupled with biomarkers to stratify patients into three risk groups. We aimed to prospectively evaluate the prespecified risk stratification tool to establish whether it can identify those at highest risk of dysplasia or cancer to prioritise the timing of endoscopy; and safely be used to follow up the low-risk group, thus sparing patients from unnecessary endoscopies.
Methods: Participants were recruited as part of two multicentre, prospective, pragmatic implementation studies from 13 hospitals in the UK. Patients with non-dysplastic Barrett's oesophagus had a capsule-sponge test which was assessed in an ISO-accredited laboratory. Patients were included if they were aged at least 18 years with a non-dysplastic Barrett's oesophagus diagnosis at their last endoscopy who were undergoing surveillance according to the published UK guidelines. Patients were assigned as low (clinical and capsule-sponge biomarkers negative), moderate (negative for capsule-sponge biomarkers, positive clinical biomarkers-age, sex, and segment length), or high risk (p53 abnormality or glandular atypia regardless of clinical biomarkers, or both). The primary outcome was a diagnosis of high-grade dysplasia or cancer necessitating treatment, according to the risk group assignment.
Findings: 910 patients recruited between August, 2020, and December, 2024 participated, of whom 138 (15%) were classified as high risk, 283 (31%) moderate risk and 489 (54%) low risk. The positive predictive value for any dysplasia or worse in the high-risk group was 37·7% (95% CI 29·7-46·4). Patients with both atypia and aberrant p53 had the highest risk of high-grade dysplasia or cancer (relative risk 135·8 [95% CI 32·7-564·0] relative to the low-risk group). The prevalence of high-grade dysplasia or cancer in the low-risk group was 0·4% (95% CI 0·1-1·6); the negative predictive value for any dysplasia or cancer was 97·8% (95% CI 95·9-98·8). Applying a machine learning algorithm as part of a digital-pathology workflow reduces the proportion needing p53 pathology review to 32% without missing any positive cases.
Interpretation: The risk-panel substantially enriches for dysplasia and capsule-sponge-based surveillance could be used in low-risk Barrett's oesophagus in lieu of endoscopy.
Funding: Innovate UK, Cancer Research UK, National Health Service England Cancer Alliance.
Copyright © 2025 The Author(s).